Table of Contents

On October 1, The Nobel Prize in Physiology or Medicine 2018 was awarded jointly to Dr. James P. Allison and Dr. Tasuku Honjo "for their discovery of cancer therapy by inhibition of negative immune regulation.
The Nobel Assembly at Karolinska Institute released the comments that Dr. Allison and Dr. Honjo showed how different strategies for inhibiting the brakes on the immune system could be used in the treatment of cancer [1-5]. The seminal discoveries by the two laureates constitute a landmark in our fight against cancer. https://www.nobelprize.org/prizes/medicine/2018/prize-announcement/
I sincerely wish to congratulate two recipients, co-workers and many patients cooperated in clinical trials. In this journal, we have published several reviews and papers on immunity checkpoints so far. The reason is that the mission of this journal is in scientific awareness and dissemination of new immunotherapy.
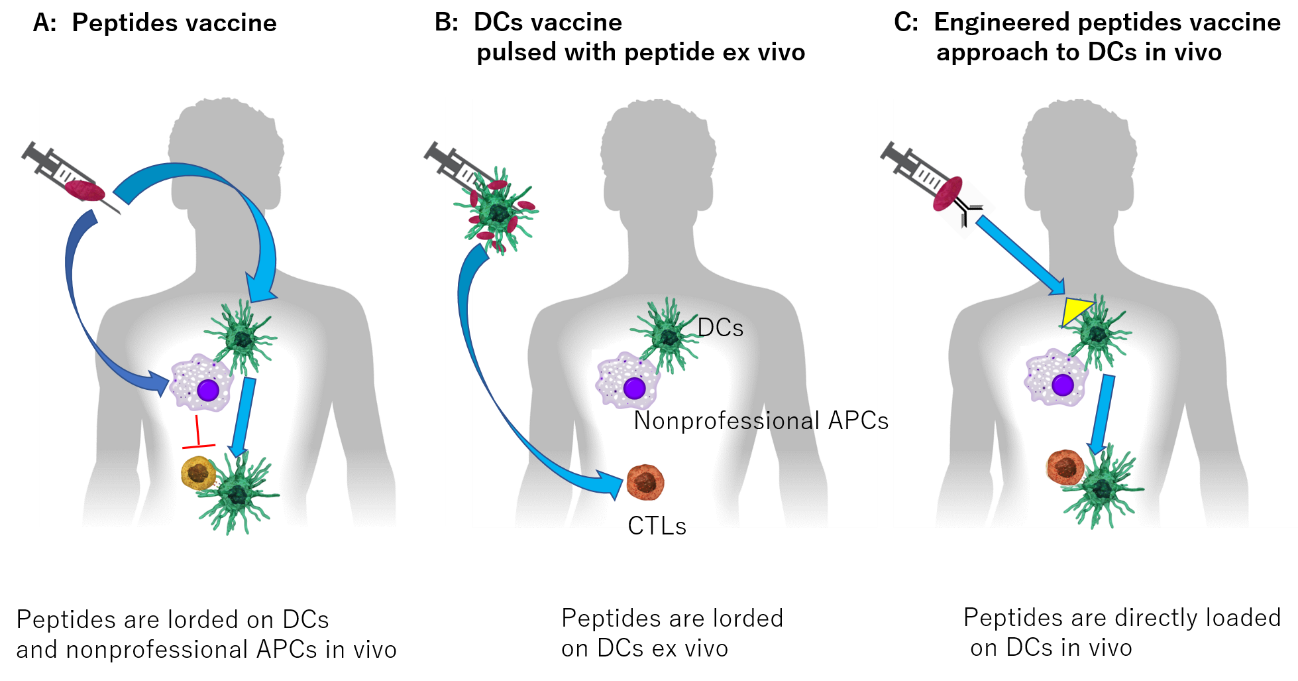
I herein introduce briefly my previous report with some modification [6]. Immune checkpoints inhibitors (ICIs) have opened promising avenues in the treatment of cancer. Various blocking antibodies targeting programmed cell death -1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) are approved for human use. They significantly improved disease outcome in a number of cancer patients by boosting anti-tumor immune responses. As Seidel, Otsuka and Kabashima described in their review article [7], mortality among advanced stage patients and the frequency of treatment-related adverse events remain high with current treatment. And, it is also noteworthy that unexpected immune related adverse effects (irAEs) appear, even when it becomes better to be administered to many cancer patients [8]. Unfortunately, the mechanisms of endocrine irAEs by ICIs, remain unclear, and optimal prevention, prediction, and treatment of the irAEs are still uncertain. However, appropriate uses and index setting related to prognosis are being studied in many fields such as dermatology [9] and other organs [6,7].
While solving such problems, we have to understand that the mechanisms of immunogenic cell death are now moving from concepts to the clinic. We will explain the mechanism behind ICD and how it will perhaps breathe a new life into chemotherapy use in cancer, not front and center but as a helpful hand to immunotherapy [10].
For a long time many scientists or doctors attempted to engage the immune system in the fight against cancer. Many clinicians stared at cancer patients who were to be dying by their side, mourning their inability. However, cancer therapy by inhibition of negative immune regulation checkpoint therapy has fundamentally changed the way we view how cancer can be managed and many patients will receive much better care and cure.
Although cancer chemotherapy has historically been considered immune suppressive, we now understand that combining chemoimmunotherapy incites a mechanism called Immunogenic cell death. These mechanisms are now moving from concepts to the clinic. Recently dramatic advances in lung cancer treatment by combining chemotherapy with immunotherapy have led the way to this new frontier in cancer medicine. We will explain the mechanism behind ICD and how it will perhaps breathe a new life into chemotherapy use in cancer, not front and center but as a helpful hand to immunotherapy.
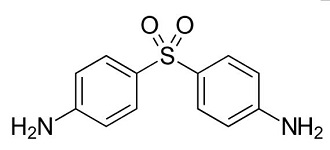
Dapsone is a synthetic sulfone preparation and is second-line treatment for cutaneous lupus erythematosus (CLE) including discoid LE (DLE). This report describes a patient with DLE, which was improved by dapsone quickly but the recurrence was noted. Oral administration of prednisolone (5 mg / day) was required but the complete healing was very difficult. In this report, we will discuss the effectiveness and limitation of dapsone for patients with DLE and the mechanisms of action.
Pyogenic arthritis, pyoderma gangrenosum, and acne (PAPA) syndrome is an autoinflammatory disease characterized by destructive inflammation of the skin and joints in association with genetic mutation of the Pombe Cdc15 homology family member PSTPIP1. Because a therapeutic strategy specific to this disease has not been established, treatment is always challenging for clinicians. We herein describe a case of PAPA syndrome with typical clinical features successfully treated with combination therapy of traditional anti-inflammatory drugs. A 39-year-old man presented with painful plaques on his extremities that had been present for several years. Large brown plaques were observed on both arms and legs with numerous fistulae and ulcers. Cystic acne lesions subsequently appeared on his cheeks and upper back. We diagnosed the patient with PAPA syndrome based on the presence of typical clinical features; however, no genetic mutations of exon-1 to 15 of PSTPIP1were found. Although recent reports have emphasized the efficacy of biologics that target inflammatory cytokines such as antibodies to interleukin-1β and tumor necrosis factor-α, use of these agents remains uncovered by health insurance in Japan, showing unresolved discrepancy in practical use for clinicians. The present patient was successfully treated with combined therapy of a corticosteroid, colchicine, and cyclosporine A, encouraging the use of this combination therapy as a novel therapeutic option.
Nakajo-Nishimura syndrome (NNS) is a very rare hereditary disorder that has its onset in infancy with pernio-like skin rashes, and is accompanied by remittent fever and nodular erythema-like skin eruptions. The treatment of NNS is still under groping. Recently we encountered a case that was treated by corticosteroid and a humanized anti-human IL-6 receptor monoclonal antibody. As a result, the fever and skin rash was not improved sufficiently, and clinical symptoms of fat atrophy and joint contracture were gradually progressing. We herein report the effects of these agents and discuss the possibilities of new treatment direction.
Voriconazole is a universal anti-fungal prophylaxis, which is frequently taken to the patients after hematopoietic stem cell transplantation and solid organ transplantation. Voriconazole can cause phototoxicity, multiple erythema in sun-exposed areas may develop actinic keratosis and cutaneous squamous cell carcinoma (cSCC) while taking voriconazole. In North America and Europe, case reports of phototoxicity and aggressive cSCC in patients on voriconazole have been documented. Also 4 cases of voriconazole-associated cSCC have recently been reported in Japan. We describe a Japanese woman with multiple cSCC associated with recurrence of cSCC after discontinuing voriconazole.

Nakajo-Nishimura syndrome (NNS) is an autosomal, recessively inherited disorder, which has been reported by Japanese physicians. This disease is characterized by remittent fever, pernio-like skin rashes, nodular erythema-like skin eruptions and partial lipodystrophy. NNS is an immunoproteasome-associated autoinflammatory disorder caused by a mutation of the PSMB8 gene. In general, autoinflammatory diseases are not associated with autoantibody production because it is assumed that autoinflammatory disorders are caused by the dysfunction of innate immunity and/or the dysfunction of proteasomes that have been collectively designated as proteasome-associated autoinflammatory syndromes. Autoinflammatory diseases were originally defined as diseases in which autoantibodies and autoreactive T cells were not detected, without activation of antigen-specific adaptive immune system, unlike autoimmune diseases. However, in recent years, as we previously reported, cases with the appearance of autoantibodies have been reported, and the boundaries are becoming vague.
We herein discuss the relationship between ANA and autoinflammatory NNS. We collected 9 cases with NNS, in which 5 cases showed positive ANA or anti-dsDNA antibody during the course.
The autoantibodies in NNS is also expected due to abnormal production and response of IFNα, but detailed pathological conditions need to be elucidated by accumulation and examination of further cases in the future. In other words, NNS will become a bridge with research on refractory autoimmune diseases.












 Open Access
Open Access