Full Issue
| View or download the full issue |
Table of Contents
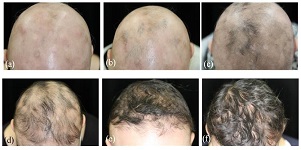
Alopecia totalis (AT) with body hair loss is the most severe type of alopecia areata (AA). The ability to develop hair is suggested to be poor in such severe AA, because AT does not respond to corticosteroid pulses and immunotherapy using squaric acid dibutylester (SADBE) or diphenylcyclopropenone (DPCP). The purpose of this study is to assess the possibility of hair regrowth in AT with body hair loss. Ten patients with AT who did not respond to topical immunotherapies, received triamcinolone acetonide (TA) injections. Undiluted or 2-fold diluted solutions of TA were prepared and 0.1–0.2 mL of either of the two solutions was administered to each patient. In total, 2 mL of the selected solution was injected monthly into each area. In cases where vellus hair developed after the injections, we restarted the immunotherapy using SADBE or DPCP and continued the therapies for more than half a year. The development of vellus hair after TA injections was defined as a good response. Complete response rate to the topical injection of TA was 10% (1/10), however the partially good response rate was 60% (6/10). The good responders showed the anagen stage of hair follicle after TA injections. Furthermore, the complete responder to TA showed susceptibility to the subsequent immunotherapy and more regrowth of hair was seen. Even if patients with AT have suffered for a prolonged period since onset, it is possible to recover the hair cycle if they show susceptibility to intralesional corticosteroid and subsequent immunotherapy.
Currently, about half of people in Japan suffer from allergic diseases. Thus, Citrus jabara fruits have been paid attention as one of quite effective anti-allergic functional foods. C. jabara is an endemic species originally grown only in Kitayama village, Wakayama prefecture in Japan. Although genetic characterization and diversity of various Citrus fruits including C. jabara were researched, but there is room for the study on flavonoids characteristics in C. jabara fruit. For the alleviation of allergic symptom, anti-inflammatory effects are also important. In this study, characteristics of flavonoids in C. jabara fruit peels, and the anti-inflammatory effects of these purified flavonoids were investigated. Our results revealed that C. jabara is a unique Citrus that almost all of flavonoids in fruit peels was narirutin. There was no Citrus species with a flavanone glycosides content ratio like C. jabara. Although anti-inflammatory effects of narirutin was weak, but its aglycone naringenin exhibited following inhibitory effects: nitric oxide synthesis (IC50 = 105 μM), nitric oxide synthase induction, Interleukin-6 synthesis (IC50 = 65 μM), and inducible soluble epoxide hydrolase activity (IC50 = 267 μM). Since narirutin is deglycosylated to naringenin that is then absorbed by colonocytes, it is considered that narirutin exists like a prodrug and its aglycone naringenin works as an active form of anti-inflammatory effect in a living body at oral ingestion of C. jabara fruit peels.
Immune checkpoint inhibitors, such as nivolumab, have been recognized that the enhanced immune responses often lead to immune-related adverse events (irAEs) in various organs. Although cutaneous toxicity is one of the most common irAEs, bullous pemphigoid (BP) with immune checkpoint inhibitors is rare. Herein, the authors report a case of BP in a patient of the metastatic malignant melanoma of the brain under the treatment of nivolumab. It is notable that this case showed the clear correlation between the status of using of nivolumab and serum levels of anti-BP180 antibody. In addition, the skin eruptions in the case were mainly pruritic erosive or crusted papules and these clinical features may be the clinical characteristics of BP induced by nivolumab.
Immune-checkpoint inhibitors (ICIs) are novel agents directed to various malignant tumors. During ICI therapy, however, immune related adverse effects (irAEs) including endocrine dysfunctions have been reported. Dysfunctions in the pituitary gland and the thyroid gland by ICI are often observed, and those in the adrenal glands and the pancreas are less frequent. Positive correlation of the prevalence of endocrine irAEs to clinical antitumor effectiveness during ICI therapy has been reported. The mechanisms of endocrine irAEs by ICI, however, remain unclear, and optimal prevention, prediction, and treatment of the irAEs are still uncertain. This review describes possible mechanisms involved in ICI-related immunity, and discusses clinical management of endocrine irAEs during ICI therapy.
Sarcoidosis is a systemic disorder with unknown etiology and pathogenesis characterized by non-caseating granulomas, and different clinical manifestations of sarcoidosis hinder diagnosis and treatment. Therefore, a comprehensive understanding of serological markers based on clinical observations of sarcoidosis and the progression of granulomas would aid analysis in routine clinical practice. In this review, we overview common serological markers, including angiotensin converting enzyme (ACE) and lysozyme, and describe in detail new promising indices in sarcoidosis such as a T cell serological marker (soluble interleukin 2 receptor; sIL-2R) and thymus and activation-regulated chemokine (TARC/CCL17).
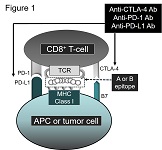
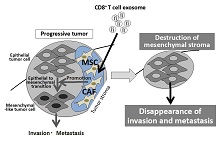
Immune system is a precise mechanism for maintenance of homeostasis by lymphocyte-mediated elimination of extracellular and intercellular pathogens, and abnormal cells in cytokine-, chemokine-, antibody-, and cytotoxic granule-dependent manners. Extracellular vesicles, e.g. exosomes, released from multivesicular endosome in immune cells have been known to be a part of the immune system. Exosomes released by antigen-presenting cells (APCs) such as macrophages and dendritic cells (DCs) regulate natural killer (NK) cells, CD8+ T cells (Cytotoxic T lymphocytes [CTLs]), and CD4+ T cells (Th cells) including Th1, Th2, and regulatory T (Treg) cells. In the anti-tumor immune system, NK cells and CTLs are mainly involved in the elimination of tumor cells by direct interaction. Recently, we clarified that tumor-infiltrating CD8+ T cells prevent tumor invasion and metastasis by exosome-mediated destruction of tumor stroma consist of mesenchymal stem cells (MSCs) and cancer-associated fibroblasts (CAFs). In this review article, we describe the role of exosomes in controlling immune system and its clinical application.












 Open Access
Open Access