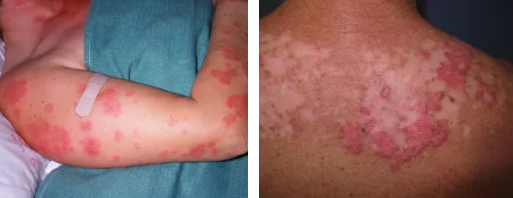
What is cutaneous lupus erythematosus? Cutaneous lupus erythematosus is an immune disease that damages the skin and leads to the emergence of some antibodies. It mostly occurs in women of childbearing age, and may also occur in children and the elderly. Comprehensive factors such as heredity, infection, environment, sex hormones and drugs can lead to immune disorder and the occurrence of the disease. Most of the lesions of skin and mucous membrane can appear various rashes. The mild ones are slightly edematous erythema, and the severe ones can appear blisters, ulcers, erosion, skin atrophy and pigmentation. Butterfly erythema is a skin and mucous membrane manifestation with diagnostic specificity. Arthritis or joint swelling and pain, keratitis may also occur. Due to genetic and environmental factors, patients have lost immune tolerance, resulting in the production of antinuclear antibodies.
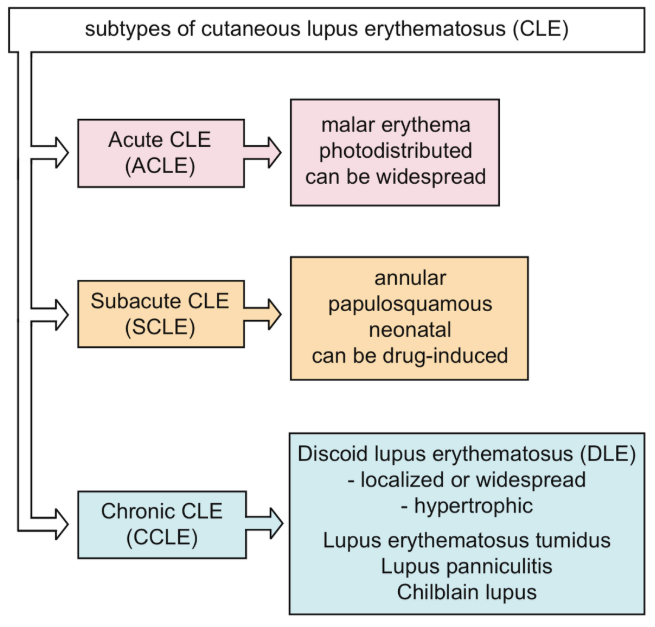
Generally, the antimalarial drug hydroxychloroquine (HCQ) is regarded as the clinical treatment of cutaneous lupus erythematosus (CLE), including all subtypes (Fig. 1), and its efficiency has been proved to be as high as 75% - 95%. Although HCQ is effective, there may be potential adverse effects (AES), including gastrointestinal symptoms and skin reactions. Rare reactions include anemia, myopathy, cardiotoxicity, agranulocytosis, hepatotoxicity and retinopathy. Retinopathy is the most widely discussed, especially after the introduction of the latest dose recommendations of HCQ[1].
Figure 1. Cutaneous lupus erythematosus (CLE) subtype Hydroxychloroquine (HCQ) was recommended as a clinical systemic therapeutic drug for cutaneous lupus erythematosus (CLE) in western countries and was approved in Japan in 2016. However, the efficacy of hydroxychloroquine on various cutaneous lupus erythematosus subtypes in Japanese patients has not been clarified[2]. Fukumi Furukawa ‘ review introduced the mechanism of development of CLE from the viewpoint of autoantibodies, cytokines, and innate immunity as well as the mechanism of HCQ[3]. For information, please click: https://systems.enpress-publisher.com/index.php/ti/article/view/1269
Source from:
|