By Jim Lucas Contributions from Ashley Hamer published February 02, 2022 When the zeroth law was originally conceived in the 18th century, there were already two laws of thermodynamics. .However, this new law, which presented a formal definition of temperature, actually superseded the existing laws and should rightfully be at the head of the list, according to OpenStax, an educational organization run by Rice University. This created a dilemma: The original laws were already well known by their assigned numbers, and renumbering them would create a conflict with the existing literature and cause considerable confusion. One scientist, Ralph H. Fowler, came up with a solution to the dilemma: He called the new law the "zeroth law." (Cambridge University Press, 1939).
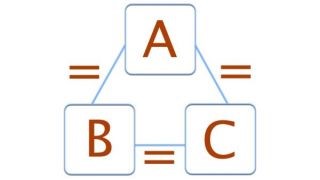
Figure1:The zeroth law of thermodynamics (Image credit: Tim Sharp) The zeroth law of thermodynamics states that if two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other. Thermal equilibrium means that when two bodies are brought into contact with each other and separated by a barrier that is permeable to heat, there will be no transfer of heat from one to the other.
Source from: 1. https://www.livescience.com/50833-zeroth-law-thermodynamics.html |